Betafix
Fistula compression bandage
Description
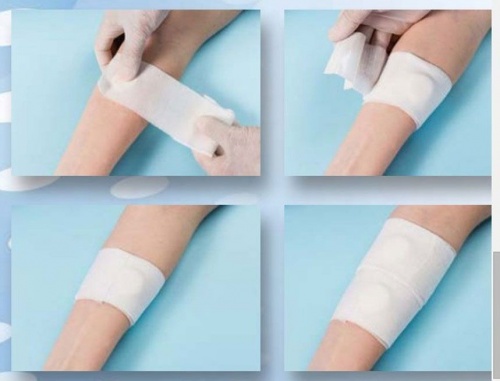
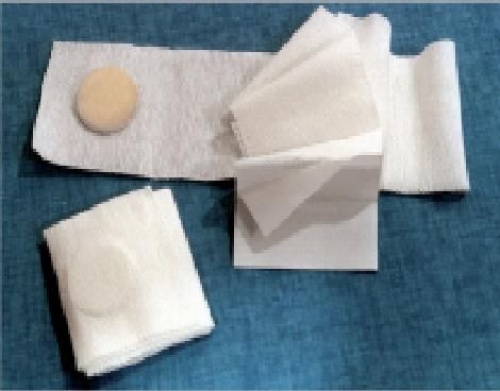
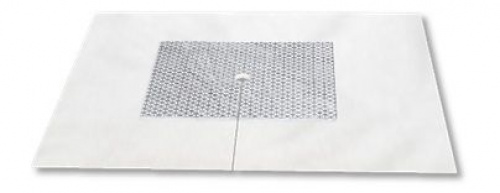
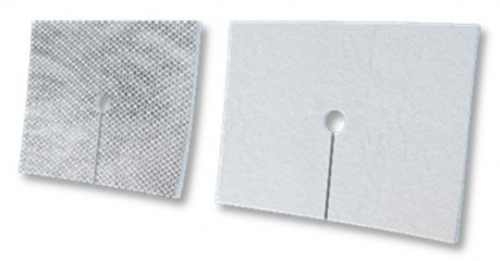
Elastic fistula compression bandage with high absorption tampon with 2 layers separated by a waterproof film and ending with a plaster to ensure the bandage to be keept in place.
Aims
Suggested for risk reducing haemorrage thanks to the continue compression on needle insertion points and acting as a protective barrier against infections. The biocompatible non-stick film on the tampon ensures a lesser adhesion on the injured skin and non traumatic peeling off.
- Caracteristics
- Detail
- Related products
| Reference | Description | Packaging |
| S0029 | Sterile blister with 1 Betafix fistula bandage | 400 |
| S0030 | Sterile blister with 2 Betafix fistula bandage | 250 |
| S0210 | Blister with 2 fistula pad (diameter 2,6cm) | 400 |
Elastic fistula bandage provided with a two layer highly absorbent pad and plaster for a better tightness.
-
Designed to stop bleeding and to reduce the haemorragic risk, thanks to the compression on the needles holes, and to work as a barrier against the risk of infection.
-
The non-adherent, biocompatible layer on the pad doesn’t stick to the skin and guarantees a non-traumatic removal.
-
The plaster helps to keep the pad in position.
Emostan
Haemostatic dressing post-dialysis
Emostan
Haemostatic dressing post-dialysis for the arteriovenous fistula.
Emostant manual compression device especially designed to compress and reduce the bleeding time.
- Easy to Use
- Preserve the fistula
- Biocompatible
- Doesn't stick to the skin
- Fewer rosk of bleeding after the dressing withdrawal
- Caracteristics
- Detail
- Related products
| References | Diameter | Thickness |
| S0210 | 2.6 cm | 8 mm |
Packaging : Box of 500 blisters containing 2 dressing.
Composition
- Dressing made of Polyethylene viscose nonwoven and Polypropylene.
Sovan
Adhesive haemostatic dressing
Adhesive dressing
Sovan is an adhesive dressing with non-adhesive and biocompatible tampon on hypoallergenic adhesive tape
- Tape : lengths of 10 or 15 cm, width of 5 cm, white color
- Tampon : 2,6 cm diameter
- Caracteristics
- Detail
- Related products
| References | Adhesive band size (cm) | Tampon diameter (cm) | Dressing / package | Package / Box |
| S0074 | 15 x 5 | 2,5 | 1 | 500 |
| S0075 | 15 x 5 | 2,5 | 2 | 500 |
| S0079 | 10 x 5 | 2,5 | 2 | 500 |
Composition
- Tape - Hypopallergenic adhesive band
- Tampon - Non woven polyethylene, polypropylene
Betafix Alginate
Alginate Fistula Bandage
BETAFIX ALGINATE
Elastic fistula bandage with multilayer haemostatic pad based on calcium alginate.
INTENDED USE
All patients with haemostasis problems in order to stop bleeding of vascular access.
These may include: elderly patients, diabetic patients, individuals with vitam K deficiency, patients treated with heparin or warfarin, patients suffering from liver disease, partients with hereditary disease (such as haemophilia and Von Willebrand disease), obese patients.
PURPOSE OF USE
- Bleeding control in patients undergoing dialysis treatment.
- Speed up of haemostasis process.
- Suitable to reduce bleeding thanks to continous compression on needles injection sites.
- Caracteristics
- Detail
- Related products
| Bandage | |||||
| Code | length (cm) | width (cm) | Pad (cm) | Pcs/Pack | Pcs/Box |
| B6006C25251A | 60 +/- 10% | 6 | 2,5 x 2,5 | 1 | 50 |
Composition
- Pad - Fiber of calcium alginate
- Elastic Bandage - Cotton-Polyamide
- Support - Polyester Non Woven 100%
- Adhesive - hypo-allergenic based on acrylates
Sterilization by Gamma irradiation.
Emostan Alginate
Fistula haemostatic pad calcium alginate based
EMOSTAN ALG
Fistula multilayer haemostatic pad based on calcium alginate.
INTENDED USE
All patients with haemostasis problems in order to stop bleeding of vascular access.
These may include: elderly patients, diabetic patients, individuals with vitam K deficiency, patients treated with heparin or warfarin, patients suffering from liver disease, partients with hereditary
disease (such as haemophilia and Von Willebrand disease), obese patients.
PURPOSE OF USE
Bleeding control in patients undergoing dialysis treatment.
Speed up of haemostasis process.
Suitable to reduce bleeding thanks to continous compression on needles injection sites.
- Caracteristics
- Detail
- Related products
| Code | Pad (cm) | Pcs/Pack | Pcs/box |
| S25251A |
2.5 x 2.5 |
1 | 50 |
| S25252A |
2.5 x 2.5 |
2 | 25 |
Composition
- Pad - Fiber of calcium alginate
- Support - Polyester
Sterilization by Gamma irradiation.
Exit-Pro SoftTouch
Catheter Dressing
EXIT-PRO AG SoftTouch
Exit-site dressing with pre-cut silver ion-releasing anti-adherent film for the catheter exit, applied on a transpiring non-woven patch coated with siliconebased hypoallergenic adhesive gel with “SoftTouch” technology.
INTENDED USE
Complete dressing of the insertion points of intravascular devices. It does not require the use of secondary dressings. Compatible with all types of catheters of standard use.
INDICATION
The dressing performs a double action:
- the silver ion-releasing film has an immediate and longlasting antimicrobial action, helping to reduce the risk of local infections of exit-site and tunnel as well as the sepsis associated with the use of intravascular devices.
- the “SoftTouch” adhesive film preserves the skin from mechanical damage caused by continuous removal of the patch (as a consequence of repeated treatments), helping tissue repair. Optimal solution for patients experiencing dry, moist or scaly skin widespread in patients undergoing, for example, dialysis treatment and oncology treatment.
- Caracteristics
- Detail
- Related products
| Code | Adhesive (cm) | Pad (cm) | Hole diameter (mm) | Pcs/pack | Pcs/box |
|
S0810/AG |
10 x 8 | 5 x 4 | 4 | 1 | 25 |
Composition
- Pad – Silver Non-woven of Polyethylene/Viscose Polypropylene
- Support – polyester
- Adhesive– hypoallergenic material based on silicone gel
Silver concentration : 0.030 mg/cm²
Weight of the pad : 185±10% g/m²
Sterilization by ethylene oxyde.
Exit-Pad Argent
Silver Dressing
Exit-Pad Argent
Non adherent silver ions based dressing.
INDICATIONS
Specific for the Exit-Site dressing.
To be used for compression and absorption of the exudates.
PURPOSE
- The silver layer has an immediate and long term antibacterial action helping to reduce the risk of local infection of the Exit-Site, of the tunnel or systemic (catheter-correlated sepsis) related to the use of intravascular devices.
- It helps to prevent irritation caused by the mechanical rubbing of the catheter over the skin.
- Caracteristics
- Detail
- Related products
| Code | Pad (cm) | Model | Indication | Pcs/pack | Pcs/box |
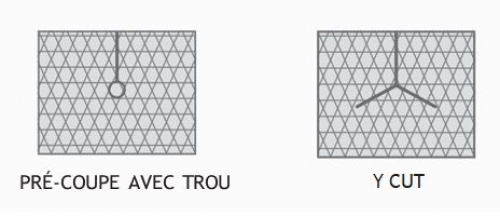
| S0903/AG | 3,5 x 4,5 | with cut and hole Ø 4 mm |
MONO-LUMEN CATHETERS | 1 | 100 |
| S0904/AG | 3,5 x 4,5 | with cut and hole Ø 5 mm |
PERITONEAL CATHETER | 1 | 100 |
| S0905/AG | 5x 4 | with Y-cut | TESIO CATHETER | 1 | 100 |
| S0925/AG | 3,5 x 2,2 | with cut and hole Ø 4 mm |
TESIO CATHETER | 2 | 100 |
Composition
- Pad – Silver Polyethylene/Viscose Non-woven Polypropylene
Silver concentration : 0.030 mg/cm²
Weight of the pad : 185±10% g/m²
Sterilization by Gamma irradiation.